* Terms and Conditions apply.
Revision sheets for IGCSE or GCSE physics. This series revision sheets are based on the IGCSE & GCSE syllabuses. Lots of formulas, definitions and key points to learn off. There are also accompanying test sheets to check your memorisation.
The idea is read the information and than print the quiz sheet and try the questions.
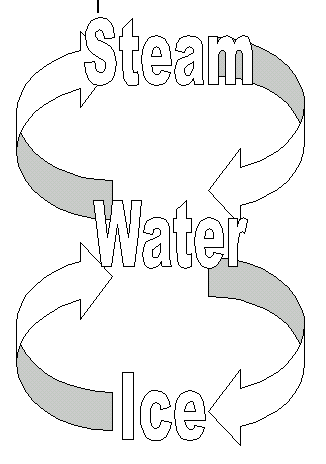
Physics exercise
Revision Exercise six:
unit of temperature : degrees Celsius (oC), kelvin (K),
unit of density kilogram/metre 3 (kg/m3)
unit of pressure, pascal (Pa)
Density and pressure
density = mass / volume
ρ = m / V
pressure = force / area
p = F / A
a substance can change state from solid to liquid by the process of melting
a substance can change state from liquid to gas by the process of evaporation or boiling
particles in a liquid have a random motion within a close-packed structure
particles in a solid vibrate about fixed positions within a close-packed regular structure
Discovering the Nucleus,
Geiger and Rutherford
(at Manchester University)
Brownian motion, is random motion caused by the random motion of air molicules.
molecules in a gas have a random motion and that they exert a force and hence a pressure on the walls of the container
there is an absolute zero of temperature which is – 273 oC
the kelvin temperature = the Celsius temperature + 273
an increase in temperature results in an increase in the speed of gas molecules
the kelvin temperature of the gas is proportional to the average kinetic energy of its molecules
for a gas in a sealed container when the kelvin temperature increases the pressure increases.
the relationship between the pressure and kelvin temperature of a fixed mass of gas at constant volume:
p1/ T1 = p2/ T2
the relationship between pressure and volume of a fixed mass of gas at constant temperature
p1V1 = p2V2
Quiz for revision exercise six: 3 stages of matter quiz